What atom has the highest oxidation number?
The highest known oxidation state is reported to be +9, displayed by iridium in the tetroxoiridium(IX) cation ( IrO+4).
What compound has the atom with the highest oxidation number?
The highest known oxidation state is +9 in the tetroxoiridium (IX).
How to know which compound has the highest oxidation number?
The highest oxidation state of an element is determined using the periodic table by the group in which it is located. Metals in all compounds have a positive oxidation state. In compounds with non-metals, hydrogen has an oxidation state of +1, and an oxidation state of -1 with metals.
Which has the highest oxidation number N?
Nitrogen can have oxidation states range from -3 (as in ammonia) to +5 (as in dinitrogen pentoxide).
Which element shows the highest oxidation state?
Mn exhibits the largest number of oxidation states because it has electronic configuration [(Ar)3d54s2], Hence maximum oxidation state of Mn is + 7. Q.
Which compounds are most oxidized?
The compounds become increasingly oxidized as we move from left to right, with each step gaining a bond to oxygen and losing a bond to hydrogen. Carbon dioxide, in which all four bonds on the carbon are to oxygen, is in the highest oxidation state.
What compound has the highest atomic number?
To date, the highest atomic number element to be discovered or synthesized is oganesson (symbol Og), with atomic number 118.
Which compound has oxidation number?
All alkali metals (group 1 elements) have an oxidation state of +1 in their compounds. All alkaline earth metals (group 2 elements) exhibit an oxidation state of +2 in their compounds. In the compounds made up of two elements, a halogen (group 17 elements) have an oxidation number of -1 assigned to them.
What is the oxidation number in most compounds?
The oxidation number of hydrogen in most compounds is +1. The oxidation number of fluorine in all compounds is −1. Other halogens usually have an oxidation number of −1 in binary compounds, but can have variable oxidation numbers depending on the bonding environment.
What is the highest oxidation number of oxygen?
The highest oxidation state of oxygen is +2 which is possible when it combines with fluorine.
Which of the following has highest oxidation power?
Fluorine is the strongest oxidizing agent because it is the strongest oxidant among all the elements.
Which compound is most prone to oxidation?
The alkane is the most reduced form of a hydrocarbon, while the alkyne is the most oxidized form. Oxidation reactions in organic chemistry often involve the addition of oxygen to a compound, which changes the particular functional group of that compound.
How do you find the highest oxidation number?
The minimum oxidation number of an element is equal to the group number (from the periodic chart) minus 8. The maximum oxidation number of an element (except elements in group IB) is equal to the group number from the periodic chart.
What are the highest and lowest oxidation numbers of N?
That means, nitrogen has five valence electrons and has a valency of 3. So, nitrogen can either accept 3 electrons or donate 5 electrons. – Therefore, the oxidation state of nitrogen ranges from -3 to +5.
Which of the following shows highest oxidation number in compound state?
Therefore, ruthenium (Ru) shows highest oxidation number i.e. +8 in combined state.
Why is the highest oxidation state?
– Therefore, the highest oxidation state of metals is exhibited by oxides and fluorides due to their high oxidizing power. Note: Remember due to high electronegativity, oxygen and fluorine have high oxidizing power and hence, highest oxidation state of metals is achieved in their oxide or fluoride form.
Which of the following has higher oxidation state?
Hence, Mn shows the maximum oxidation state.
Which metal has the highest oxidation?
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4. (ii) Zirconium and Hafnium exhibit similar properties.
Which atom is most easily oxidized?
The order of some common metals in the electromotive series, starting with the most easily oxidized, is: lithium, potassium, calcium, sodium, magnesium, aluminum, zinc, chromium, iron, cobalt, nickel, lead, hydrogen, copper, mercury, silver, platinum, gold.
Which has the highest oxidizing property?
Because of its abundance in the atmosphere, oxygen gas (O2) is the most common oxidizing agent. Fluorine (F) is the most strong oxidizing agent, and some Halogens are also strong oxidizing agents.
Which compound is easiest to oxidize?
Answer and Explanation: Lithium is an alkali metal. Alkalis are easily oxidized. The reduction potential of Lithium is very low and the oxidation potential of Lithium is very high. Lithium actively reacts with oxygen to form Lithium oxide.
Is element 119 possible?
Ununennium, also known as eka-francium or element 119, is a hypothetical chemical element; it has symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element has been discovered, confirmed, and a permanent name is decided upon.
Which has the highest number of compounds?
Carbon forms the maximum number of compounds in the periodic table. Since carbon has the property of tetra valency and catenation so they can form a maximum number of compounds. Carbon exists in several allotropic forms.
What is the rarest element?
Astatine is a chemical element; it has symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth’s crust, occurring only as the decay product of various heavier elements.
What is the highest oxidation number of oxygen?
The highest oxidation state of oxygen is +2 which is possible when it combines with fluorine.
How do you find the highest oxidised group?
We can predict the most positive oxidation state of an element from the number of valence electrons it has. Positive oxidation states occur from the loss of electrons in an element. So the most positive oxidation state of an element would be the largest number of electrons the element can lose.
What is the highest oxidation state of element with atomic number 23?
Five outermost electrons are there in the element of atomic number 23 and the element is named Vanadium. It can lose maximum five electrons to attain stable electronic configuration and thus it has the highest oxidation number +5.
Which metal has the highest oxidation?
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4. (ii) Zirconium and Hafnium exhibit similar properties.
Which atom has the highest oxidation number?
Which element has the highest oxidation state in x2o7?
What does oxidation number mean in chemistry?
Which element has an oxidation state of +1?
Oxidation Numbers: A Quick Refresher
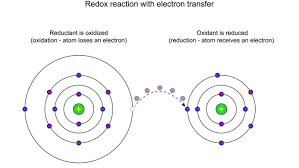
Remember, oxidation numbers are all about keeping track of electrons in chemical reactions. They tell us how many electrons an atom has gained or lost in a chemical bond. A positive oxidation number means an atom has lost electrons, while a negative oxidation number means it’s gained electrons.
Think of it like this: A positive oxidation number is like being in debt – you’ve lost something!
The Big Players
Now, when it comes to super high oxidation numbers, we’re looking at elements that are really good at losing electrons. These are your highly electronegative elements, like oxygen and fluorine.
Let’s break down some key contenders:
Osmium Tetroxide (OsO4): Here, the osmium atom has a whopping +8 oxidation number! That’s a serious amount of electron loss!
Xenon Tetroxide (XeO4): Another powerhouse! Xenon in this compound rocks a +8 oxidation number as well.
Ruthenium Tetroxide (RuO4): Not to be outdone, ruthenium in this compound also boasts a +8 oxidation number.
So, we’ve got three compounds, each with a +8 oxidation number for their central atom. But hold on! This doesn’t mean they all tie for first place.
The Winner, By a Hair
It seems like there’s a slight edge to OsO4 (Osmium Tetroxide) when it comes to the highest oxidation number. Why? Because osmium is known to be able to reach +8 a bit more readily than xenon or ruthenium.
But Wait, There’s More!
While we’ve found the current champion, the world of chemistry is always evolving! We might stumble upon a new compound or even discover a way to push an element even further in terms of electron loss.
FAQs
Q: Why is it important to know about oxidation numbers?
A: Oxidation numbers are fundamental in chemistry. They help us understand how atoms behave in reactions, predict the types of chemical bonds they form, and even balance chemical equations.
Q: What are some real-world applications of oxidation numbers?
A: Oxidation numbers play a role in a wide range of applications, from batteries and fuel cells to corrosion prevention and environmental monitoring.
Q: Can oxidation numbers be fractional?
A: You bet! In certain compounds, like superoxides and peroxides, atoms can have fractional oxidation numbers. This happens when the electrons are shared unevenly.
Let me know if you have any other questions. Happy learning!
See more here: What Compound Has The Atom With The Highest Oxidation Number? | Which Compound Has The Atom With The Highest Oxidation Number
Which compound has the atom with the highest oxidation
Textbook Question. For each of the following balanced oxidation–reduction reactions, (i) identify the oxidation numbers for all the elements in the reactants and products and (ii) Pearson
Oxidation States (Oxidation Numbers) – Chemistry
The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation state) or Chemistry LibreTexts
5.3: Oxidation number – Chemistry LibreTexts
Oxygen has an oxidation number of +2 because the single oxygen atom has “gained” a total of two electrons, one from each hydrogen. Here is another molecule Chemistry LibreTexts
Oxidation Numbers – Chemistry | Socratic
Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Assigning these numbers involves several rules: Free atoms (H2) usually Socratic
SOLVED: Which compound has the atom with the highest
to determine which compound has the element with the highest oxidation number, We need to assign oxidation numbers for all the elements and all the Numerade
11.1: Oxidation Numbers – Chemistry LibreTexts
The oxidation number of oxygen in most compounds is \(-2\). The oxidation number of hydrogen in most compounds is \(+1\). The oxidation number of fluorine in all Chemistry LibreTexts
Oxidation Number | Brilliant Math & Science Wiki
The sum of oxidation numbers of all atoms in a given compound is zero (for a neutral compound), or equal to the charge on the compound (for compounds that have a Brilliant
How to Find Oxidation Number & Oxidation State
In an ionic compound with 2 elements, the metal or the more electronegative atom has a positive oxidation number, and the non-metal or less electronegative atom has a ChemTalk
See more new information: pilgrimjournalist.com
Which Compound Has The Atom With The Highest Oxidation Number? A) Mgso4 B) Na3N C) Cas D) Al(No2)3 …
How To Calculate Oxidation Numbers – Basic Introduction
Iit/Jee Chemistry Practice #5: Oxidation Numbers
How To Find Oxidation Numbers (Rules And Examples)
Song Of Chemistry 😂😂 #Science #Chemistry #Songs
Link to this article: which compound has the atom with the highest oxidation number.
See more articles in the same category here: https://pilgrimjournalist.com/wiki/