Why is N in pyrrole SP2?
In pyrrole, the nitrogen has four electron groups but the ring constraints and the hope for aromaticity make it energetically more favourable to have the ideal $ s{p^2} $ hybridisation instead of $ s{p^3} $ . The third $ s{p^2} $ orbital is used to bond with the hydrogen.
How to find hybridization of nitrogen in pyrrole?
The hybridization of nitrogen in pyrrole is $s{p^2}$ hybridization as the lone pair of nitrogen are in conjugation (with double bond) and further making it an aromatic compound.
What is the hybrid state assumed by N in pyrrole?
The nitrogen in pyrrole contributes two pi electrons by becoming sp2 hybridized and placing its lone pair electrons into a p orbital.
Is N sp2 hybridized?
The nitrogen atom also hybridizes in the sp2 arrangement, but differs from carbon in that there is a “lone pair” of electron left on the nitrogen that does not participate in the bonding.
Why is sp3 n more basic than sp2 n?
If we keep that in mind, then we can rank them as follows, the Nitrogen on the left is sp3 hybridized making it the most basic because it would be the least acidic and the Nitrogen on the right, because it hybridizes to sp2 is going to be less basic base on hybridization.
How does hybridization of carbon and nitrogen in pyrrole differ?
Each carbon in pyrrole contributes one pi electron. The nitrogen in pyrrole contributes two pi electron by becoming sp2 hybridized and placing its lone pair electrons into a p orbital.
What is the hybridization of the N in?
The nitrogen is sp3 hybridized which means that it has four sp3 hybrid orbitals. Two of the sp3 hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H sigma bonds.
How do you identify sp2 hybridization?
Ch 2: sp2 hybridisation. When a C atom is attached to 3 groups and so is involved in 3 σ bonds, it requires 3 orbitals in the hybrid set. This requires that it is sp2 hybridised.
What is the NH in pyrrole?
The NH proton in pyrroles is moderately acidic with a pKa of 17.5. Pyrrole can be deprotonated with strong bases such as butyllithium and sodium hydride. The resulting alkali pyrrolide is nucleophilic. Treating this conjugate base with an electrophile such as iodomethane gives N-methylpyrrole.
What is the hybridization of the N in this amine?
The nitrogen atom in most amines is sp3 hybridized.
Is pyrrole sp2 or sp3?
In the protonated pyrrole, the nitrogen atom is bonded to four different atoms (two carbon atoms and two hydrogen atoms), requiring sp3 hybridization and leaving no unhybridized p orbital.
What is the hybridization of N in piperidine structure?
N in piperidine has three σ bond and one lone pair of electrons, hence N is sp3 hybridised.
Why is N sp3 hybridization?
nitrogen utilizes sp3 hybridization to achieve an optimal trigonal planar geometry as it has a non-bonding lone pair when its formal charge is neutral. We know that carbon sp3 hybridizes to be able to form three -bonds; in its non-hybridized state, carbon only allows two bonds.
What is the hybridization of nitrogen in pyridine?
The correct option is B sp2, trigonal planar.
What is the hybridization of N in aniline?
Short Answer. Answer: The hybridization of nitrogen in aniline is sp².
How do you know if N is sp2 or sp3?
To determine hybridization, you count the number of atoms that are bonded to the atom and add this number to the number of lone pairs there are around the atom. sp goes with 2, sp2 goes with 3, etc.
Is pyridine more basic than pyrrole?
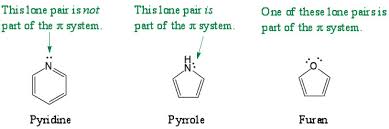
Note: In simple words, we can say that the lone pair of nitrogen in pyrrole will be in resonance, so it cannot be donated, whereas the lone pair of nitrogen in pyridine is localized and hence, it can easily donate hydrogen ions. Therefore, pyridine is more basic than pyrrole.
Which n is more basic?
Increasing order of N atom basicity The lone pair electron on nitrogen-1 is localized and it is s p 3 hybridized. So, nitrogen-1 is the most basic.
What is the basicity of pyridine?
Interestingly, the heterocyclic amine having a nitrogen atom as part of an aromatic ring has much less basicity than its corresponding alicyclic counterpart. For this reason, as presented in Figure 1, piperidine (pKb = 2.8) is significantly more basic than pyridine (pKb = 8.8).
Why is pyrrole planar?
In pyrrole, the nitrogen atom exhibits the presence of one lone pair, and 3 sigma bonds. But, pyrrole is aromatic. Not just that, the lone pair of nitrogen is delocalised. So, the nitrogen, in fact, exhibits a planar geometry.
What is the bonding of pyrrole?
Pyrrole is a five-membered heterocyclic organic compound having the general formula C4H4NH. It consists of two pi bonds and one lone pair of electrons contributing to a pi system. Thus it has six pi electrons.
What is the orbital structure of pyrrole?
Pyrrole is a five-membered heterocyclic ring which has 5 p orbitals and six pi electrons contributing to its aromaticity. Each carbon in pyrrole contributes one p orbital and pi electron.
Why is N sp2 hybridised?
Why is N sp2 hybridized? Its because only sigma bond pairs and lone pairs are contained by the hyberidised orbitals and not the π bond pair . So in this case nitrogen has 1 lone pair and 2 sigma bonds .
What is the hybridization of the N in NO+?
Hybridization of N in NO+ Marked atom is sp hybridized because it has lone electron pair and one σ bond that are sp hybridized and one π bond that is formed by overlapping p orbitals. Therefore, nitrogen has sp hybridization.
What is the hybridization of N in cyanide?
Following this, the hybridisation schemes of C and N in the cyanide anion are sp for both. sp hybridization . The triple bond could indicate that the hybridization of both carbon and nitrogen is sp. …
Why is nitrogen in amide sp2?
First, there is a partial double bond between the carbonyl carbon atom and the nitrogen atom of the amide. Second, the nitrogen atom is sp2 hybridized, because otherwise you could not have the 2p orbital to take part in the pi electron delocalization.
Why do aromatic amines show sp2 hybridization?
We are correctly taught that the nitrogen in simple aliphatic amines is pyramidal (sp3 hybridized). However in aniline, due to the resonance interaction between the aromatic ring and the nitrogen lone pair, considerable flattening of the nitrogen occurs (if it were completely flat it would be sp2 hybridized).
Why is sp2 called sp2?
sp2 hybridization is also called trigonal hybridization. It involves mixing of one ‘s’ orbital and two ‘p’ orbital’s of equal energy to give a new hybrid orbital known as sp2.
Why does N2 have sp hybridization?
Re: Hybridization of N2 It has a triple bond and one lone pair on each nitrogen atom. When determining hybridization, you must count the regions of electron density. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.
Is pyrrole sp 2 hybridized?
Why is nitrogen sp2 hybridized in pyrrole?
Does pyrrole have a lone pair electron?
Are pyrrole and imidazole sp2 hybridized?
Understanding Hybridization in Pyrrole
Imagine a nitrogen atom in its ground state. It has 5 valence electrons, right? Now, when it’s part of a pyrrole molecule, it needs to form bonds with its neighboring carbon atoms and a hydrogen atom. To make this happen, its orbitals need to rearrange themselves, and that’s where hybridization comes in.
Think of hybridization as a special mixing of atomic orbitals. In pyrrole, the nitrogen atom undergoes sp2 hybridization. What does that mean? Well, one s orbital and two p orbitals combine to form three new hybrid orbitals, called sp2 orbitals. These sp2 orbitals are arranged in a trigonal planar geometry, meaning they point towards the corners of an equilateral triangle.
Important Note: The remaining p orbital on the nitrogen atom remains unhybridized and is perpendicular to the plane of the sp2 orbitals. This unhybridized p orbital is crucial for the aromatic character of pyrrole.
The Role of Nitrogen in Pyrrole’s Aromaticity
Let me break down why hybridization is so important for pyrrole’s aromatic nature:
1. Structure: The sp2 hybridization of the nitrogen atom in pyrrole results in a planar structure. All five atoms in the ring lie in the same plane.
2. Delocalization: The unhybridized p orbital on the nitrogen atom overlaps with the p orbitals of the adjacent carbon atoms. This overlap creates a continuous pi system above and below the plane of the ring.
3. Aromaticity: This continuous pi system is crucial for pyrrole’s aromatic nature. Aromaticity is a special kind of stability that results from the delocalization of electrons in a cyclic system. This delocalization gives pyrrole unique properties, including its resistance to addition reactions.
Why is Hybridization Important?
Now you might be wondering, “Why should I even care about hybridization? What difference does it make?”
Well, hybridization has a huge impact on the properties of pyrrole:
Bond Angles: The sp2 hybridization of the nitrogen atom in pyrrole leads to bond angles of approximately 120 degrees, giving the molecule its planar geometry.
Reactivity: The aromatic nature of pyrrole due to hybridization makes it relatively unreactive to addition reactions.
Acidity: You’ll often see pyrrole described as a weak acid. This is because the lone pair of electrons on the nitrogen atom is partially delocalized into the pi system, making it less available for protonation.
The Bottom Line
The hybridization of the nitrogen atom in pyrrole is a vital aspect of its structure and reactivity. Understanding hybridization helps us predict its properties and reactivity, which is important for understanding the roles pyrrole plays in various chemical reactions and biological systems.
FAQs
Let’s address some common questions about hybridization in pyrrole:
1. Why doesn’t the nitrogen in pyrrole undergo sp3 hybridization like in amines?
Good question! In amines, the nitrogen atom typically undergoes sp3 hybridization, leading to a tetrahedral geometry. However, in pyrrole, the nitrogen atom needs to contribute its lone pair of electrons to the pi system to achieve aromaticity. This is only possible if it undergoes sp2 hybridization.
2. What is the effect of hybridization on the bond length in pyrrole?
Sp2 hybridization leads to shorter C-N bond lengths in pyrrole compared to C-N bonds in amines. This is because sp2 hybridized orbitals have a higher s-character, making the bonds stronger and shorter.
3. Can pyrrole act as a Lewis base?
Yes, although pyrrole is a weak base, it can still act as a Lewis base due to the lone pair of electrons on the nitrogen atom. However, this lone pair is partially delocalized into the pi system, making it less available for donation.
4. How is the hybridization of nitrogen in pyrrole different from that of nitrogen in pyridine?
In pyridine, the nitrogen atom is sp2 hybridized, but it does not contribute its lone pair of electrons to the pi system. This is because the nitrogen atom in pyridine has a lone pair in an sp2 hybrid orbital which is not involved in the pi system. This makes pyridine a stronger base than pyrrole.
5. How can I visualize the hybridization of nitrogen in pyrrole?
You can use molecular modeling software or online tools to visualize the hybridization of nitrogen in pyrrole. These tools will show you the shapes and orientations of the orbitals involved in the hybridization process.
6. What are some applications of pyrrole and its derivatives?
Pyrrole and its derivatives have numerous applications, including:
Pigments and dyes: Pyrrole derivatives are used in the synthesis of pigments like indigo and porphyrins.
Pharmaceuticals: Pyrrole derivatives are found in medications like antibiotics and anti-cancer agents.
Polymers: Pyrrole derivatives can be used in the synthesis of polymers like polyacetylene and polypyrrole.
Understanding hybridization in pyrrole opens up a whole new world of possibilities for exploring its structure, properties, and applications. It’s a fundamental concept that helps us understand the behavior of this fascinating heterocyclic compound.
See more here: How To Find Hybridization Of Nitrogen In Pyrrole? | Hybridisation Of N In Pyrrole
15.5: Aromatic Heterocycles – Pyridine and Pyrrole
The nitrogen in pyrrole contributes two pi electrons by becoming sp 2 hybridized and placing its lone pair electrons into a p orbital. The electrostatic potential map of pyrrole show the Chemistry LibreTexts
1 What is the hybridisation of nitrogen in pyrrole,
The hybridisation and the geometry of nitrogen atom in pyridine is: Q. Assertion :Pyridine is more basic than pyrrole. Reason: Lone pair of electrons on N in pydrine and pyrrole are different in nature, these BYJU’S
15.5 Aromatic Heterocycles: Pyridine and Pyrrole – OpenStax
In pyrrole, each of the four sp 2-hybridized carbons contributes one π electron and the sp 2-hybridized nitrogen atom contributes the two from its lone pair, which occupies a p OpenStax
15.6: Aromatic Heterocycles – Pyridine and Pyrrole
Pyrrole (spelled with two r’s and one l) and imidazole are five-membered heterocycles, yet both have six π electrons and are aromatic. In pyrrole, each of the four sp 2-hybridized Chemistry LibreTexts
1.10: Hybridization of Nitrogen, Oxygen, Phosphorus and Sulfur
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H Chemistry LibreTexts
Single-site pyrrolic-nitrogen-doped sp2-hybridized
Here, we report the preparation of single pyrrolic N-doped carbon materials (SPNCMs) with a tunable nitrogen content from 0 to 4.22 at.% based on a strategy of Nature
Aromatic heterocycles II (video) | Khan Academy
We’ll start with pyrrole right down here. The pyrrole molecule, as you can see, five atoms in the ring, and if we take a look at the carbons in the ring, we can see that those carbons all have a double bond to them. Therefore, each of those carbons is sp two Khan Academy
Resonance structures and hybridization (video) | Khan
We know that the lone pair is held within a hybridized sp2 orbital because the double bond connected to the nitrogen has a pi bond (i.e. the unhybridized p orbital) which must contain a pair of electrons used to form the double bond. Khan Academy
Organic Chemistry II – Ankara Üniversitesi
The pyrrole nitrogen atom is sp2. hybridized, and its unhybridized p orbital overlaps with the p orbitals of the carbon atoms to form a continuous ring. The lone pair on nitrogen Ankara Üniversitesi Açık Ders Malzemeleri
See more new information: pilgrimjournalist.com
The Hybridization States Of The Nitrogen Atom In Pyridine Piperidine And Pyrrole Are Respectively
The Hybridization States Of The Nitrogen Atom In Pyridine Piperidine And Pyrrole Are Respectivel…
A Closer Look At Pyrrole, Furan, And Thiophene
Hybridization In Pyrrole And Ammonia L Jee Mains L Neet L B.Sc. Iiird Year
Special Case Of Hybridizations In Nitrogen Compounds.
Link to this article: hybridisation of n in pyrrole.
See more articles in the same category here: https://pilgrimjournalist.com/wiki/