What is the role of crown ether in phase transfer catalyst?
The oxygen atoms are well situated to coordinate with a cation located at the interior of the ring, whereas the exterior of the ring is hydrophobic. The resulting cations often form salts that are soluble in nonpolar solvents, and for this reason crown ethers are useful in phase transfer catalysis.
How does 18-crown-6 work as a phase transfer catalyst?
18-Crown-6 binds to a variety of small cations, using all six oxygens as donor atoms. Crown ethers can be used in the laboratory as phase transfer catalysts. Salts which are normally insoluble in organic solvents are made soluble by crown ether.
How do crown ethers act as PTC?
The efficiency of Crown ethers as phase transfer catalyst depends on their complex cation constant with the reaction ion pairs,their ability to enlarge the interionic pair distance and the lipophilicity of the ion pair complexes.In general, the PTC reaction describes a methodology for accelerating the reaction between …
What is the crown ether?
A “crown ether ” is a cyclic ether containing several (i.e., 4, 5, 6 or more) oxygen atoms. It is possible to dissolve ionic compounds in organic solvents using crown ethers. Cyclic polyether with four or more oxygen atoms separated by two or three carbon atoms.
What is the role of crown ethers in drug delivery?
In general, it can be concluded that the presence of crown ethers in the process of drug delivery enhance the penetration and solubility of the drugs. The role of crown ethers as drug carriers is quantitatively calculated using specific parameters (86).
What is the role of crown ethers in nucleophilic substitution reaction?
Answer and Explanation: Crown ether tightly binds cations in a complex. These cations could be present in salts that are soluble or dissolve in non-polar solvents. Therefore, crown ethers are used as phase transfer catalysts in nucleophilic substitution and other reactions.
What is the role of phase-transfer catalyst?
Phase transfer catalyst (PTC) is used to transfer the desirable active form of an anion from the aqueous phase to organic phase where the reaction occurs.
What is the difference between Dibenzo 18-crown-6 and 18-crown-6?
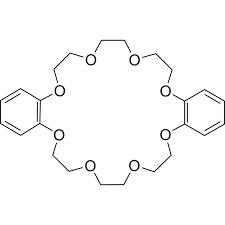
Dibenzo-18-crown-6 can be synthesized from catechol and bis(chloroethyl) ether. In contrast to those of 18-crown-6, complexes of the dibenzo crown are flattened, which often allows higher coordination numbers at the encapsulated metal cation.
What is 18-crown-6 in organic synthesis?
The compound known as 18-crown-6 is one of the simplest and most useful of the macrocyclic polyethers. Its synthesis in low yield was first reported by Pedersen. Greene6 and Dale and Kristiansen7 have reported syntheses of the title compound from triethylene glycol and triethylene glycol di-p-toluenesulfonate.
How do you calculate crown ether?
Crown ethers have the general formula of (OCH2CH2)n or (OCH2CH2CH2)n and are named using both the total number of atoms in the ring and the number of oxygen atoms. Thus 18-crown-6 is an 18-membered ring with six oxygen atoms.
What is the role of crown ether in organic synthesis?
(Because of their remarkable selectivity, crown compounds can find and wrap around a guest atom in a solution.) Crown ethers are commonly utilized in organic synthesis. Crown ethers can help with phase transfer catalysis. Crown ethers are commonly used in complex cations, amines, phenols, and other organic compounds.
Are crown ethers hydrophobic?
Crown ethers (CEs) are macrocyclic polyethers and have three to twenty oxygen atoms alienated by two or more carbon atoms. These macromolecules can be either substituted or unsubstituted. This class of organic compounds has an interesting structure with a hydrophobic ring surrounding a hydrophilic cavity [1].
Are crown ethers soluble in water?
Crown ethers containing cyclohexyl have a higher solubility than the corresponding benzo crown ether in water, alcohols and aromatic hydrocarbons, also in petroleum ether. Crown ether generally has a good thermal stability. However its ether bonds are susceptible to oxidation when melted or at a high temperature.
What is the biological importance of crown ethers?
Crown ethers are supramolecular receptors5 that play a crucial role in the formation of host–guest complexes. Their complexing ability has found applications in catalysis (including phase-transfer catalysis, PTC6), transport of metal cations through membranes,7 and the synthesis of catenanes8 and rotaxanes.
What are crown ethers and cryptates?
➢ Crown ethers (or crowns) are known as a group of macrocyclic polyethers. Many. macropolycyclic ligands which are related to each other are also known to us and are called as ‘cryptates’ (or cryptands or simply, crypts). ➢ The two rings of cryptand provide extra strength to hold the ion.
What are crown ethers good for?
Crown ethers are effective complexing agents for alkali metal ions, ammonium ions, and water. Crown ethers generally activate enzymes, such as subtilisin, chymotrypsin, and lipase, in organic media, and consequently enhance the reactivity and enantioselectivity of these enzymes.
What is the application of crown ethers in extraction?
Crown ethers have high selectivity and affinity towards specific metals. So they are used as potent extracting agents for removing alkali metal salts18 from mixture.
What is the crown ether for sodium ion?
15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+); however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.
What is the role of ether in the reaction?
Ether is also used as a solvent since it reacts with Grignard reagents forming a stable complex. The Magnesium-halogen bond is ionic and solvates the carbon-oxygen bond of ether thus forming a stable complex and increases the ability of the Grignard reagent to react.
What is the method of synthesis of crown ether?
In principle, co-polymerization of a 1,2-diol and a 1,2-dihalide might lead to a polyether. One molecule of base is consumed for each new C–O bond made, and these reactions terminate quickly before long chains are made. It is more useful for making the cyclic oligomers called ‘crown ethers’.
Why does ether act as a nucleophile?
It is etheral oxygen with two lp of electrons that attacks conc. acid(protonic) to form oxonium salts. Attacks acids (lewis acids like BF3, AlCl3 etc) to form complexes. Hence , we can say that it as like a nucleophile.
What are the classification of phase-transfer catalysts?
PTC reactions can be broadly classified into two main classes: soluble PTC and insoluble PTC (Figure 1). Within each class, depending on the actual phases involved, reac- tions are further classified as liquid-liquid PTC (LLPTC), gas-liquid PTC (GLPTC), and solid-liquid PTC (SLPTC).
What is a phase transfer?
When your child moves from one stage of education to another this is called a phase transfer and happens when your child moves from: early years setting to school. infant school to junior school. primary to secondary school. secondary school onto post-16 or 19 provision.
What are the phase-transfer catalysts for oxidation reactions?
Phase-transfer catalysis (PTC) offers many excellent opportunities for conducting oxidation reactions using inexpensive primary oxidants such as oxygen, sodium hypochlorite, hydrogen peroxide, electrooxidation, permanganate, periodic acid, and others.
What is the meaning of 18 crowns?
In the past, 18 crowns refer to 18 monkey warriors who were once 18 gods volunteering to help Narai when he was reborn as Pra Ram. These 18 monkeys have different names. The reason why the idiom “18 crowns” conveys a negative meaning is that, during the reign of King Rama 6, there was a notorious group of gamblers.
What is 18-crown-6 crystal structure?
The asymmetric unit of this structure contains two independent cation–anion pairs of the title complex, [K(18-crown-6)][Co(η3-C7H7)(η5-C7H9)], where 18-crown-6 stands for 1,4,7,10,13,16-hexaoxacyclooctadecane (C12H24O6), in general positions and well separated.
What is the application of crown ethers in extraction?
Crown ethers have high selectivity and affinity towards specific metals. So they are used as potent extracting agents for removing alkali metal salts18 from mixture.
What is the role of ether in the reaction?
Ether is also used as a solvent since it reacts with Grignard reagents forming a stable complex. The Magnesium-halogen bond is ionic and solvates the carbon-oxygen bond of ether thus forming a stable complex and increases the ability of the Grignard reagent to react.
What do crown ethers have the capability of forming?
Crown-ethers are macrocyclic polyethers capable of forming host-guest complexes, especially with inorganic and organic cations.
What is the biological importance of crown ethers?
Crown ethers are supramolecular receptors5 that play a crucial role in the formation of host–guest complexes. Their complexing ability has found applications in catalysis (including phase-transfer catalysis, PTC6), transport of metal cations through membranes,7 and the synthesis of catenanes8 and rotaxanes.
Are crown ethers a phase-transfer catalyst?
Can crown ethers be used as catalysts for asymmetric catalysis?
What is a crown ether-catalyzed phase-transfer reaction?
Can crown ethers be used for chiral catalysis?
So, you’re looking to learn about phase transfer catalysis and crown ethers? You’re in the right place! These are fascinating tools used in organic chemistry to make reactions happen smoother and more efficiently.
Let me break it down for you. Imagine you have two ingredients for a delicious chemical reaction, but they don’t like to mix. One ingredient is in water, and the other is in oil – they’re like oil and water, right? That’s where phase transfer catalysts, like crown ethers, come in to play. They act like a friendly bridge, allowing those ingredients to react and create something awesome.
What are Crown Ethers?
Think of crown ethers as tiny, ring-shaped molecules with oxygen atoms arranged like a crown. This special structure allows them to bind to metal ions, forming a complex that can dissolve in organic solvents. That’s the magic! This allows them to move metal ions from a water-based environment into an organic environment, bridging the gap between the two phases.
Crown Ethers as Phase Transfer Catalysts
Crown ethers are powerful phase transfer catalysts because they make it easy for reactions to happen in the presence of a hydrophilic (water-loving) and hydrophobic (water-hating) environment. They can help reactions proceed faster and with higher yields.
Now, let’s talk about some popular crown ethers and their uses:
18-Crown-6: This is a classic, and it’s super good at binding potassium ions. It’s often used in reactions that require a potassium base, like in the Williamson ether synthesis where it can help create ethers.
15-Crown-5: This one has a smaller ring, so it binds to sodium ions best. It’s a handy tool for reactions that need a sodium base.
How Crown Ethers Work
Let’s imagine a reaction where a potassium hydroxide (KOH) base is needed. KOH is a strong base, but it doesn’t easily dissolve in organic solvents. But, 18-Crown-6 can help!
1. 18-Crown-6 forms a complex with potassium ions, making them soluble in organic solvents.
2. This complex then reacts with the organic substrate, allowing the reaction to proceed smoothly.
Benefits of Using Crown Ethers
Why are crown ethers such a big deal? Here’s the lowdown:
Improved Yields: They can increase reaction yields by facilitating the transfer of reactants between phases.
Faster Reaction Rates: Crown ethers can speed up reactions by bringing the reactants together in the right environment.
Milder Reaction Conditions: Sometimes, reactions can be done at lower temperatures or with less harsh conditions when using crown ethers.
Enhanced Selectivity: They can improve the selectivity of reactions, meaning they can help produce the desired product with fewer side products.
Examples of Crown Ether Applications
Crown ethers are versatile! Here are a few examples:
Organic Synthesis: They are used in a wide range of organic reactions, such as Williamson ether synthesis, alkylation, and nucleophilic substitution reactions.
Polymer Chemistry:Crown ethers are helpful in synthesizing polymers with unique properties.
Analytical Chemistry: They are used in ion sensing and extraction processes.
Safety Considerations
Just like with any chemical, it’s important to be aware of the safety precautions when working with crown ethers.
Skin and Eye Contact: They can cause skin and eye irritation, so it’s important to wear appropriate safety gear, like gloves and goggles.
Fire Hazard: Some crown ethers are flammable, so handling them near open flames or heat sources should be avoided.
FAQs
Q: What are the main differences between crown ethers and other phase transfer catalysts?
A:Crown ethers are known for their high selectivity towards specific metal ions, especially alkali metals like potassium and sodium. They are often favored in reactions involving these ions.
Q: How do I choose the right crown ether for my reaction?
A: The choice of crown ether depends on the size of the metal ion you want to complex and the specific requirements of your reaction. Consult a reliable resource, like a chemistry textbook or online databases, for guidance on selecting the right crown ether.
Q: Are there any alternatives to crown ethers for phase transfer catalysis?
A: Yes! Other types of phase transfer catalysts include quaternary ammonium salts and phosphonium salts. These catalysts can be used for different applications and might be more suitable depending on the specific reaction conditions.
Q: What’s the future of crown ethers in chemistry?
A: Crown ethers are still a valuable tool in many fields, and researchers are constantly finding new and exciting applications for them. They hold a lot of potential for creating new materials and technologies.
Overall, crown ethers are a fascinating and powerful tool in organic chemistry. By understanding how they work and their applications, you can harness their potential to create efficient and effective reactions.
See more here: How Does 18-Crown-6 Work As A Phase Transfer Catalyst? | Phase Transfer Catalyst Crown Ether
Angewandte Chemie International Edition – Wiley Online Library
The MOF-CE C can be instantaneously suspended in immiscible two-phase solvents as an effective phase-transfer catalyst and can form various uniform membranes with enhanced adsorption and separation performance, which highlights the Wiley Online Library
Supramolecular asymmetric catalysis mediated by crown
Applications of catalytic crown ethers, even their pseudorotaxanes and rotaxanes derivatives, as chiral catalysts in organic reactions sprang up subsequently, in ScienceDirect
Direct synthetic routes to functionalised crown ethers
The general approach for the introduction of moieties on aliphatic crown ethers involves a radical mediated cross dehydrogenative coupling initiated either by photochemical or RSC Publishing
Cation-Controlled Catalysis with Crown Ether-Containing
Fundamental studies of cation–crown interactions helped reveal the potential utility of crown ethers in applications ranging from phase transfer catalysis to separations science and RSC Publishing
Crown ethers as phase-transfer catalysts. A comparison
Anion-promoted nucleophilic substitutions carried out in aqueous–organic two-phase systems in the presence of catalytic amounts of perhydrodibenzo-18-crown-6 follow the RSC Publishing
Theoretical Design and Calculation of a Crown Ether
Abstract. Fluorinated organic molecules are playing an increased role in the area of pharmaceuticals and agrochemicals. This fact demands the development of efficient catalytic fluorination ACS Publications
Crown Ethers and Phase Transfer Catalysis in Polymer
Phase transfer catalysis or interfacial catalysis is a syn thetic technique involving transport of an organic or inorganic salt from a solid or aqueous phase into an organic liquid where reaction with an organic-soluble Springer
Phase-transfer catalyst – Wikipedia
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Wikipedia
Molecules | Free Full-Text | Synthesis of Novel
Based on our results, we anticipate that a linker between the crown ether and the squaramide unit may potentially increase the enantioselectivity of this new type of phase-transfer catalyst, as two MDPI
Perfluorocarbon Soluble Crown Ethers as Phase Transfer
It was clearly shown that properly designed fluoroponytailed crown ethers can promote the disintegration of the crystal lattice of alkali salts and transfer anions Wiley Online Library
See more new information: pilgrimjournalist.com
Phase Transfer Catalyst. Crown Ether.
Crown Ethers As Phase Transfer Catalyst
Phase Transfer Catalysis||Crown Ethers||Cryptates||Example||Organic Chemistry||Msc 1St Semester
Phase Transfer Catalyst – Quaternary Ammonium Salt – Organic Chemistry
Crown Ethers
Link to this article: phase transfer catalyst crown ether.
See more articles in the same category here: https://pilgrimjournalist.com/wiki/