Which metals can liberate hydrogen from acids?
Since Na Fe and Pb occur above hydrogen in the activity series they will liberate hydrogen from dilute acids.
What metals can replace hydrogen?
Here sodium, potassium, iron and lead are placed above hydrogen thus they can replace hydrogen from acids. Gold and silver are noble metals and do not react with acids.
Which metal is displaced by metal from acid?
Answer: Active metals can displace hydrogen ions from acids due to their high reactivity.
Can Zn displace H2 from acid?
Zinc can easily displace hydrogen from dilute Sulphuric acid but copper cannot.
Which metal can displace hydrogen from acid?
Hence, Sodium and Potassium are two metals that can displace hydrogen from dilute acids to form metal salts and hydrogen gas.
Why can’t copper liberate H2 from acid?
Copper is less active than Hydrogen and so it cannot replace it from acids and so it cannot liberate hydrogen with dilute acids.
What are the 4 metals that will not replace hydrogen in an acid?
Cu, Au, Ag and Pt are metals which can’t displace hydrogen from an acid. The reason for such a property is that these metals are placed lower in than hydrogen in the reactivity series. Since, they are less reactive than hydrogen, they cannot displace hydrogen from its compound.
Can gold replace hydrogen from an acid?
Copper, Gold, Silver and Platinum are less reactive than hydrogen and hence do not displace it when coming in contact with an acid.
What metal Cannot replace hydrogen in HCl?
Metals such as copper, silver, gold and platinum are less reactive than hydrogen. Hence, they cannot displace hydrogen from dil. HCl.
Can silver displace hydrogen from acid?
Hence, the two metals which are unable to displace Hydrogen are Copper and Silver.
Why do metals replace hydrogen from acids?
Metals have a tendency to lose electrons and hence they supply electrons i.e. they are electrondonors. That is why metals displace hydrogen from dilute acids. On the other hand non-metal is an electron acceptor. It cannot supply electrons to H+ and hence it does not displace hydrogen from dilute acids.
Can copper displace hydrogen?
Copper has positive electrode potential; hence it cannot displace hydrogen from dilute acids. But a number of other elements in 3d series except displace hydrogen from dilute acids.
Can nickel displace hydrogen in an acid?
Answer and Explanation: Nickel lies above hydrogen in the reactivity series while silver and copper lies below hydrogen. So only nickel is more reactive than hydrogen and thus can displace hydrogen in an acid.
Can lithium displace hydrogen from acids?
any element which is higher is the series than H+, will have the ability to displace hydrogen ion from an acid. for examples, zinc metal, lithium, sodium metal, iron.
Why does zinc replace hydrogen?
Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. Zinc quickly interacts with acid to form hydrogen bubbles.
Can zinc displace hydrogen from acid?
In electrochemical series, position of Zn and iron is above hydrogen where as copper and mercury are placed below hydrogen. Hence, Zn and Fe can replace hydrogen from dilute acids whereas copper and mercury do not.
What metal displaces hydrogen from HCL?
(b) Magnesium and aluminium are two metals that can displace hydrogen from dilute hydrochloric acid.
Can you displace hydrogen?
Note: Highly reactive metal like potassium, sodium and calcium displaces hydrogen from dilute acids with explosive violence, moderately reactive metals like magnesium, aluminium and zinc displaces hydrogen vigorously and metals such as copper, gold, mercury and silver do not displaces hydrogen from dilute acids.
Which metal cannot liberate H2 from acid?
Copper (Cu) cannot liberate H2 from dilute hydrochloric acid because it is below hydrogen in the electrochemical series.
Why does Cu not replace hydrogen from acids?
Copper and lead cannot displace hydrogen in acid because they are less reactive when compared to hydrogen.
Can you liberate hydrogen from acids?
Cu can not liberate hydrogen from acids because it has positive electrode potential. Metal having negative value of electrode potential liberate H 2 gas.
Which metals Cannot produce hydrogen from acids?
Since copper is less reactive than hydrogen, it is not able to displace hydrogen from dilute acids. The other metals being more reactive will displace hydrogen.
Which metal will replace hydrogen?
Iron and aluminium will displace hydrogen from dilute acids as they are more reactive then hydrogen.
Why non-metals Cannot displace hydrogen from the acid?
The reason why non-metals do not displace hydrogen from dilute acids is because unlike metals, non-metals do not have a tendency to lose electrons but to gain electrons. … Only those metals which are reactive than hydrogen will displace H2 from acids. Q.
Which metal cannot replace H2 from hydrochloric acid?
Copper cannot displace hydrogen from the reaction with dilute hydrochloric acid. The reason is that in electrochemical series copper is placed below the hydrogen. So, its electrode potential is less than the hydrogen.
Can barium displace hydrogen?
barium would displace hydrogen gas from cold water.
Can magnesium displace hydrogen from water?
For example, calcium is quite reactive with water, whereas magnesium does not react with cold water but does displace hydrogen from steam.
Which metal releases hydrogen with acids?
Zinc metal, reacts with both acid and base to produce hydrogen gas.
Which metal gives off hydrogen?
Metals which are more reactive than hydrogen in the reactivity series can react with acid and produce hydrogen gas. Such metals are:—potassium, sodium, calcium, magnesium, aluminium, zinc, iron etc.
Do acids liberate hydrogen with active metals?
Acids react with active metals to liberate hydrogen. Q. Sodium metal reacts with hydrochloric acid and liberate hydrogen.
Do all acids give off hydrogen with any metal?
Many, but not all, metals react with acids. Hydrogen gas isformed as the metal reacts with the acid to form a salt. Many, but not all, metals react with acids. Hydrogen gas is formed as the metal reacts with the acid to form a salt.
Which metals will displace hydrogen from an acid?
Which metal can displace hydrogen from water?
Can a metal displace hydrogen?
Can a metal displace H2 from acidic solutions?
You know how sometimes you mix things together and something totally new happens? Well, that’s what happens when you mix certain metals with acids. It’s like a chemical magic trick!
You see, some metals are really good at displacing hydrogen from acids. It’s all about their reactivity – how much they want to react with something else.
Think of it like a tug-of-war. The metal is on one side, and the hydrogen is on the other side, both pulling on the acid. The metal that wins the tug-of-war will displace the hydrogen and create a brand new substance!
So, which metals win this tug-of-war?
Well, let’s dive into the exciting world of metal reactivity and find out!
The Reactivity Series: Your Guide to Metal Mayhem
The reactivity series is our secret weapon. It’s like a list of metals, ranked by how much they love to react. The metals at the top are the most reactive, and they’re the ones who can push hydrogen out of the way.
Here’s the general idea:
Metals higher on the reactivity series are more reactive and can displace hydrogen from acids.
Metals lower on the reactivity series are less reactive and can’t displace hydrogen from acids.
Here’s a simplified version of the reactivity series:
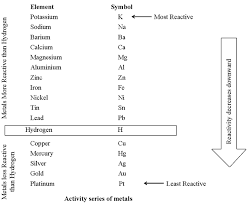
Most Reactive
1. Potassium (K)
2. Sodium (Na)
3. Lithium (Li)
4. Calcium (Ca)
5. Magnesium (Mg)
6. Aluminium (Al)
7. Zinc (Zn)
8. Iron (Fe)
9. Lead (Pb)
10. Hydrogen (H)
11. Copper (Cu)
12. Silver (Ag)
13. Gold (Au)
14. Platinum (Pt)
Least Reactive
Remember: This is a simplified version, and there are other factors that can influence reactivity.
The Great Hydrogen Displacement: How It Happens
Let’s imagine we have zinc (Zn) and hydrochloric acid (HCl).
1. Zinc is higher on the reactivity series than hydrogen.
2. This means zinc is more eager to react than hydrogen.
3. When zinc is added to hydrochloric acid, it displaces the hydrogen.
4. This means the hydrogen leaves the acid and forms hydrogen gas.
The chemical equation looks like this:
Zn + 2HCl → ZnCl2 + H2
Zinc (Zn) reacts with hydrochloric acid (HCl).
Zinc chloride (ZnCl2) is formed, and hydrogen gas (H2) is released.
Metals That Displace Hydrogen From Acids
Now that we understand the basics, let’s focus on the metals that can displace hydrogen from acids:
Group 1 Metals (Alkali Metals): These guys are the superstars of reactivity. They can easily displace hydrogen from acids like hydrochloric acid (HCl) and sulfuric acid (H2SO4). They include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
Group 2 Metals (Alkaline Earth Metals): These metals are also quite reactive. They can displace hydrogen from acids, but not as readily as the alkali metals. These include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Aluminium (Al): Aluminium is an interesting case. It forms a protective layer of aluminium oxide (Al2O3) which slows down its reaction with acids. However, if you use a strong acid like hydrochloric acid (HCl), it can still displace hydrogen.
Zinc (Zn), Iron (Fe), and Lead (Pb): These metals are less reactive than the alkali metals and alkaline earth metals, but they can still displace hydrogen from acids.
Metals That Don’t Displace Hydrogen From Acids
Here are the metals that are too shy to displace hydrogen:
Copper (Cu), Silver (Ag), and Gold (Au): These metals are less reactive than hydrogen and won’t displace it from acids. They’re like the introverts of the metal world!
Platinum (Pt): Similar to gold and silver, platinum is a very unreactive metal and won’t displace hydrogen.
It’s Not Just About The Metal!
While the reactivity series is a great guide, it’s not the only factor that determines whether a metal can displace hydrogen. Here’s a bit more detail:
The Concentration of the Acid: A more concentrated acid will have a higher concentration of hydrogen ions, making it more likely that a metal can displace hydrogen.
The Temperature: Increasing the temperature speeds up the reaction rate, which can help a metal displace hydrogen from an acid.
Safety First!
Working with acids can be dangerous, so remember these safety tips:
Always wear appropriate safety gear, including goggles and gloves.
Work in a well-ventilated area.
Never mix acids with other chemicals unless you know what you’re doing.
If you get acid on your skin, rinse it immediately with plenty of water.
FAQs:
Q: What is the product formed when a metal displaces hydrogen from an acid?
A: The product formed is a salt and hydrogen gas. For example, when zinc reacts with hydrochloric acid, the products are zinc chloride (a salt) and hydrogen gas.
Q: What happens when a metal reacts with an acid?
A: When a metal reacts with an acid, a chemical reaction takes place where the metal displaces hydrogen from the acid. This results in the formation of a salt and the release of hydrogen gas.
Q: What are some examples of metals that displace hydrogen from acids?
A: Some examples include sodium (Na), magnesium (Mg), aluminium (Al), zinc (Zn), and iron (Fe).
Q: What is the role of hydrogen in this reaction?
A: Hydrogen is the element that is being displaced from the acid by the more reactive metal.
Q: Why is it important to know which metals can displace hydrogen from acids?
A: Knowing this information is crucial for understanding chemical reactions, predicting the products of reactions, and performing safe experiments.
Q: Is there a way to test if a metal can displace hydrogen from an acid?
A: Yes, you can perform a simple experiment by adding a small piece of the metal to a solution of the acid. If bubbles of hydrogen gas are released, then the metal can displace hydrogen from the acid.
Remember, experimenting with acids should always be done under the guidance of a qualified professional and with proper safety precautions.
Now you’re ready to tackle the world of metal reactivity and hydrogen displacement!
See more here: What Metals Can Replace Hydrogen? | Which Metals Can Displace Hydrogen From Acids
Which metals will displace hydrogen from an acid in the … – Socratic
Thus, the list shows 14 metals that will displace hydrogen from acids. The video shows an experiment to determine the placement of three different metals (Cu, Zn and Mg) on the activity series. Socratic
Reactivity Series of Metals Chart, Features, Uses – BYJU’S
Despite being a non-metal, hydrogen is often included in the reactivity series since it helps compare the reactivities of the BYJU’S
P3: Activity Series of Metals – Chemistry LibreTexts
26 rows Less active metals like iron or zinc cannot displace hydrogen from water but Chemistry LibreTexts
Name two metals which will displace hydrogen from dilute acids
Metals that are more reactive than hydrogen displace it from dilute acids. For example: sodium and potassium. Metals that are less reactive than hydrogen do not displace it. Toppr
Name two metals which will displace hydrogen from dilute acids,
To understand the metals that can displace hydrogen from dilute acids, and the metals that cannot, we have to compare the reactivities of the respective metals to that of hydrogen BYJU’S
The metal which can displace hydrogen from acid is: – Toppr
Calcium (Ca) and on the other hand the metals that come below hydrogen in the reactivity series for eg. Copper (Cu), Silver (Ag), Gold (Au) are less reactive than hydrogen and Toppr
Metals and displacement reactions – Obtaining and using
Only metals above hydrogen in the reactivity series will react and displace hydrogen from acids. For example: Zinc + sulfuric acid → zinc sulfate + hydrogen BBC
In the activity series of metals, which metal(s) will
All of the elements above Hydrogen in the reactivity series will displace Hydrogen atoms from an acid during a reaction. This is because they are higher in the series than Hydrogen, hence more Socratic
The metal which can displace hydrogen from acid is: – Toppr
The metal which can displace hydrogen from acid is: A. copper. B. silver. C. calcium. D. gold. Solution. Verified by Toppr. In the reactivity series, the metals that come above Toppr
See more new information: pilgrimjournalist.com
Metals Which Can Displace Hydrogen From Acids \U0026 Which Cannot| Chemistry-X|Digital Generation.
Name Two Metals Which Can Displace Hydrogen From Acids. | 11 | Hydrogen | Chemistry | Dinesh Pu…
Name Two Metals Which Can Displace Hydrogen From Acids.
Why Do Metals Displace Hydrogen From Dilute Acids And Water?
Metals React With Acids To Produce Salt And Hydrogen | Acid \U0026 Bases | Chemistry
Gcse Chemistry Revision \”Acids Reacting With Metals\”
Gcse Chemistry – Reactivity Series Of Metals \U0026 Displacement Reactions #37
Composition Engineered Electrocatalysts For Water Splitting And Metal-Ion Batteries
Exploring Materials For Overall Electrochemical Water Splitting Activity
Metals In Acid
Link to this article: which metals can displace hydrogen from acids.
See more articles in the same category here: blog https://pilgrimjournalist.com/wiki