More Coverage: Read our examination of the factors that made the fires so destructive and our analysis, which found that at least 1,800 buildings were destroyed in Santa Rosa.


Fires destroyed an area of the Coffey Park neighborhood in Santa Rosa,
Calif. Before: Google Earth; After: California Highway Patrol, via Reuters
Hundreds of reghters have been trying to contain blazes across Northern
California’s wine country since Sunday night in what ofcials said was one of
the most destructive re emergencies in the state’s history.
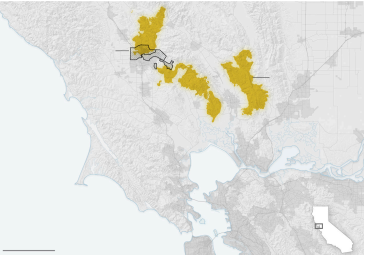
Calistoga
CALIFORNIA
Evacuation
areas as of
Thursday afternoon
St. Helena
Santa
Rosa
Extent of res,
as of Thursday
Napa
Sonoma
Petaluma
Pacic
Ocean
Novato
San Pablo
Bay
Area of
detail
Oakland
CALIFORNIA
San Francisco
25 MILES
Sources: NASA MODIS, Santa Rosa Fire Department
In Santa Rosa, the largest city in Sonoma County, hundreds of homes and
businesses were destroyed. In the Coffey Park neighborhood, bare trees dotted
the landscape where dozens of homes had been reduced to ash and rubble.
Multiple evacuation orders were issued in the city after res rst broke out on
Sunday.

Smoke and re engulfed both sides of Fountaingrove Parkway in Santa Rosa.
Fires also raged further east through the heart of Sonoma and Napa wine
country, damaging thousands of buildings and establishments, including
multiple vineyards, wineries and at least one dairy


Smoke rising behind homes in Santa Rosa. Before: Google Earth; After:
California Highway Patrol, via Reuters


A pool in front of the re-ravaged Signorello Estate winery in Napa,
Calif. Before: James Gateley; After: Marcio Jose Sanchez/Associated Press


Damaged buildings at Stornetta Dairy in Sonoma, Calif. Before: Google Street
View; After: Stephen Lam/Reuters
The res, which began Sunday night, appeared to catch residents off guard,
with some eeing on foot.
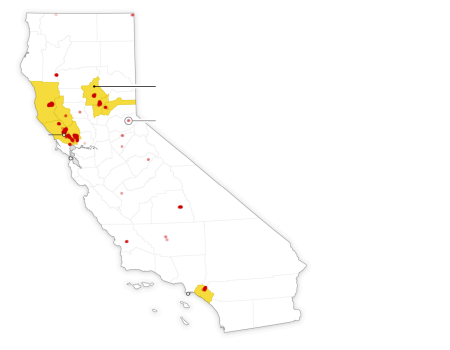
Counties with
declared states
of emergency
Fires on Monday
Santa
Rosa
San
Francisco
CALIFORNIA
Los Angeles
Sources: NASA MODIS, Ofce of the Governor
About 140,000 acres had burned by Wednesday afternoon, destroying
thousands of structures and forcing the evacuation of more than 20,000
people, ofcials said

Chronicle, via Associated Press
At least 21 people had been killed as of Wednesday afternoon. Eleven deaths
were in Sonoma County, while six people died in Mendocino County, two in
Napa County, and two in Yuba County.

About two miles northeast of Coffey Park, dozens of homes were destroyed in
the Fountaingrove area of Santa Rosa. At least some of the buildings of the
Fountaingrove Inn and the Santa Rosa Hilton, shown below, were burned to
the ground.


Buildings were destroyed in the Fountaingrove area of Santa Rosa. Before:
Google Street View; After: Justin Sullivan/Getty Images
While the cause of the res remained under investigation on Monday, ofcials
said that the blazes spread rapidly because of wind gusts of over 50 miles per
hour. Dry vegetation and low humidity contributed to the intensity of the res.


The Fountaingrove Round Barn, a local landmark built in 1899, was burned to
the ground. Before: Google Street View; After: Kent Porter/The Press Democrat,
via Associated Press


